Clip Art Vesicles Clip Art of Cell Wall in Plant Cells
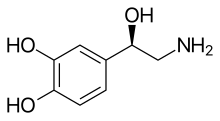 Skeletal formula of noradrenaline | |
 Ball-and-stick model of the zwitterionic form of noradrenaline found in the crystal construction[1] | |
| Clinical information | |
|---|---|
| Other names |
|
| Physiological data | |
| Source tissues | locus coeruleus; sympathetic nervous organisation; adrenal medulla |
| Target tissues | system-wide |
| Receptors | α1, α2, β1, β3 |
| Agonists | sympathomimetic drugs, clonidine, isoprenaline |
| Antagonists | Tricyclic antidepressants, beta blockers, antipsychotics |
| Precursor | dopamine |
| Biosynthesis | dopamine β-monooxygenase |
| Metabolism | MAO-A; COMT |
| Identifiers | |
| IUPAC proper name
| |
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.000.088 |
| Chemic and physical data | |
| Formula | C 8 H xi N O 3 |
| Molar mass | 169.180 g·mol−1 |
| 3D model (JSmol) |
|
| SMILES
| |
| InChI
| |
Norepinephrine (NE), also chosen noradrenaline (NA) or noradrenalin, is an organic chemical in the catecholamine family that functions in the brain and torso as both a hormone and neurotransmitter. The name "noradrenaline," derived from Latin roots meaning "at/alongside the kidneys," is more commonly used in the United Kingdom; in the United States, "norepinephrine," derived from Greek roots having that same meaning, is normally preferred.[two] "Norepinephrine" is also the international nonproprietary proper name given to the drug.[three] Regardless of which name is used for the substance itself, parts of the torso that produce or are affected by it are referred to every bit noradrenergic.
The general function of norepinephrine is to mobilize the brain and body for action. Norepinephrine release is lowest during sleep, rises during wakefulness, and reaches much college levels during situations of stress or danger, in the and so-called fight-or-flight response. In the brain, norepinephrine increases arousal and alertness, promotes vigilance, enhances formation and retrieval of memory, and focuses attention; it also increases restlessness and anxiety. In the residue of the trunk, norepinephrine increases heart rate and blood force per unit area, triggers the release of glucose from free energy stores, increases claret flow to skeletal muscle, reduces blood flow to the gastrointestinal system, and inhibits voiding of the float and gastrointestinal motility.
In the encephalon, noradrenaline is produced in nuclei that are small yet exert powerful effects on other encephalon areas. The most important of these nuclei is the locus coeruleus, located in the pons. Exterior the brain, norepinephrine is used every bit a neurotransmitter by sympathetic ganglia located near the spinal cord or in the abdomen, as well every bit Merkel cells located in the peel. It is also released directly into the bloodstream past the adrenal glands. Regardless of how and where it is released, norepinephrine acts on target cells by binding to and activating adrenergic receptors located on the cell surface.
A diversity of medically important drugs work by altering the actions of noradrenaline systems. Noradrenaline itself is widely used every bit an injectable drug for the handling of critically low claret pressure. Beta blockers, which counter some of the effects of noradrenaline by blocking their receptors, are ofttimes used to treat glaucoma, migraine, and a range of cardiovascular issues. Alpha blockers, which counter a unlike ready of noradrenaline furnishings, are used to care for several cardiovascular and psychiatric atmospheric condition. Alpha-2 agonists oft have a sedating outcome and are commonly used every bit anesthesia-enhancers in surgery, as well as in treatment of drug or alcohol dependence. Many of import psychiatric drugs exert stiff effects on noradrenaline systems in the encephalon, resulting in side-furnishings that may be helpful or harmful.
Construction [edit]
Norepinephrine is a catecholamine and a phenethylamine.[4] Its construction differs from that of epinephrine only in that epinephrine has a methyl group attached to its nitrogen, whereas the methyl group is replaced by a hydrogen atom in norepinephrine.[4] The prefix nor- is derived as an abbreviation of the word "normal", used to indicate a demethylated chemical compound.[five]
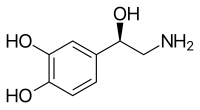
Norepinephrine structure
Epinephrine structure

Catechol structure
Biochemical mechanisms [edit]
Biosynthesis [edit]
Norepinephrine is synthesized from the amino acid tyrosine by a series of enzymatic steps in the adrenal medulla and postganglionic neurons of the sympathetic nervous system. While the conversion of tyrosine to dopamine occurs predominantly in the cytoplasm, the conversion of dopamine to norepinephrine by dopamine β-monooxygenase occurs predominantly inside neurotransmitter vesicles.[nine] The metabolic pathway is:
- Phenylalanine → Tyrosine → L-DOPA → Dopamine → Norepinephrine[9]
Thus the direct precursor of norepinephrine is dopamine, which is synthesized indirectly from the essential amino acid phenylalanine or the non-essential amino acid tyrosine.[ix] These amino acids are found in almost every protein and, as such, are provided by ingestion of protein-containing food, with tyrosine existence the virtually mutual.
Phenylalanine is converted into tyrosine by the enzyme phenylalanine hydroxylase, with molecular oxygen (O2) and tetrahydrobiopterin as cofactors. Tyrosine is converted into Fifty-DOPA past the enzyme tyrosine hydroxylase, with tetrahydrobiopterin, O2, and probably ferrous fe (Atomic number 26ii+) as cofactors.[9] Conversion of tyrosine to Fifty-DOPA is inhibited by Metyrosine, a tyrosine analog. L-DOPA is converted into dopamine by the enzyme effluvious 50-amino acid decarboxylase (as well known as DOPA decarboxylase), with pyridoxal phosphate every bit a cofactor.[9] Dopamine is so converted into norepinephrine past the enzyme dopamine β-monooxygenase (formerly known every bit dopamine β-hydroxylase), with O2 and ascorbic acid as cofactors.[9]
Norepinephrine itself can further be converted into epinephrine by the enzyme phenylethanolamine N-methyltransferase with S-adenosyl-L-methionine as cofactor.[9]
Deposition [edit]
In mammals, norepinephrine is rapidly degraded to diverse metabolites. The initial step in the breakdown can exist catalyzed by either of the enzymes monoamine oxidase (mainly monoamine oxidase A) or COMT.[ten] From there the breakdown can proceed by a variety of pathways. The principal end products are either Vanillylmandelic acid or a conjugated form of MHPG, both of which are thought to be biologically inactive and are excreted in the urine.[11]

Norepinephrine degradation.[11] Metabolizing enzymes are shown in boxes.
Functions [edit]
Cellular effects [edit]
| Family | Receptor | Blazon | Mechanism |
|---|---|---|---|
| Blastoff | αone | 1000q-coupled. | Increase IP3 and calcium by activating phospholipase C. |
| αii | Gi/Mo-coupled. | Decrease cAMP by inhibiting adenylate cyclase. | |
| Beta | β1 | Grands-coupled. | Increase army camp by activating adenylate cyclase. |
| βtwo | |||
| βthree |
Like many other biologically active substances, norepinephrine exerts its effects by binding to and activating receptors located on the surface of cells. 2 broad families of norepinephrine receptors have been identified, known as alpha and beta adrenergic receptors.[11] Alpha receptors are divided into subtypes αi and αii; beta receptors into subtypes β1, β2, and βthree.[11] All of these function as G protein-coupled receptors, meaning that they exert their furnishings via a complex second messenger system.[eleven] Alpha-ii receptors usually have inhibitory furnishings, simply many are located pre-synaptically (i.e., on the surface of the cells that release norepinephrine), and so the net result of blastoff-2 activation is frequently a decrease in the corporeality of norepinephrine released.[11] Alpha-1 receptors and all three types of beta receptors usually have excitatory effects.[11]
Storage, release, and reuptake [edit]

Norepinephrine (labeled "noradrénaline" in this drawing) processing in a synapse. Afterward release norepinephrine can either exist taken up once again by the presynaptic final, or broken down by enzymes.
Inside the brain norepinephrine functions every bit a neurotransmitter, and is controlled past a fix of mechanisms common to all monoamine neurotransmitters. Later on synthesis, norepinephrine is transported from the cytosol into synaptic vesicles by the vesicular monoamine transporter (VMAT).[12] VMAT can be inhibited past Reserpine causing a decrease in neurotransmitter stores. Norepinephrine is stored in these vesicles until it is ejected into the synaptic crevice, typically later an action potential causes the vesicles to release their contents straight into the synaptic crevice through a procedure called exocytosis.[11]
Once in the synapse, norepinephrine binds to and activates receptors. After an action potential, the norepinephrine molecules apace become unbound from their receptors. They are and so captivated back into the presynaptic prison cell, via reuptake mediated primarily by the norepinephrine transporter (NET).[thirteen] One time back in the cytosol, norepinephrine can either be broken down by monoamine oxidase or repackaged into vesicles by VMAT, making it available for hereafter release.[12]
Sympathetic nervous system [edit]

Schema of the sympathetic nervous organization, showing the sympathetic ganglia and the parts of the body to which they connect.
Norepinephrine is the primary neurotransmitter used by the sympathetic nervous system, which consists of near two dozen sympathetic concatenation ganglia located next to the spinal cord, plus a prepare of prevertebral ganglia located in the chest and abdomen.[14] These sympathetic ganglia are continued to numerous organs, including the eyes, salivary glands, center, lungs, liver, gallbladder, tummy, intestines, kidneys, urinary bladder, reproductive organs, muscles, peel, and adrenal glands.[14] Sympathetic activation of the adrenal glands causes the role called the adrenal medulla to release norepinephrine (as well as epinephrine) into the bloodstream, from which, functioning as a hormone, information technology gains further access to a broad variety of tissues.[14]
Broadly speaking, the effect of norepinephrine on each target organ is to modify its land in a way that makes it more than conducive to active body move, often at a cost of increased energy utilisation and increased wear and tear.[15] This tin can be contrasted with the acetylcholine-mediated furnishings of the parasympathetic nervous system, which modifies near of the aforementioned organs into a land more conducive to rest, recovery, and digestion of nutrient, and usually less costly in terms of energy expenditure.[15]
The sympathetic effects of norepinephrine include:
- In the eyes, an increase in production of tears, making the eyes more moist,[xvi] and pupil dilation through wrinkle of the iris dilator.
- In the heart, an increase in the corporeality of blood pumped.[17]
- In brown adipose tissue, an increase in calories burned to generate body estrus (thermogenesis).[18]
- Multiple effects on the immune organization. The sympathetic nervous system is the primary path of interaction betwixt the allowed arrangement and the brain, and several components receive sympathetic inputs, including the thymus, spleen, and lymph nodes. However the effects are complex, with some immune processes activated while others are inhibited.[19]
- In the arteries, constriction of claret vessels, causing an increase in blood pressure.[xx]
- In the kidneys, release of renin and retention of sodium in the bloodstream.[21]
- In the liver, an increase in product of glucose, either by glycogenolysis after a repast or by gluconeogenesis when nutrient has not recently been consumed.[21] Glucose is the body's principal energy source in most conditions.
- In the pancreas, increased release of glucagon, a hormone whose main event is to increase the production of glucose by the liver.[21]
- In skeletal muscles, an increment in glucose uptake.[21]
- In adipose tissue (i.e., fat cells), an increment in lipolysis, that is, conversion of fat to substances that can be used direct as energy sources by muscles and other tissues.[21]
- In the stomach and intestines, a reduction in digestive activity. This results from a generally inhibitory effect of norepinephrine on the enteric nervous system, causing decreases in gastrointestinal mobility, blood flow, and secretion of digestive substances.[22]
Noradrenaline and ATP are sympathetic co-transmitters. It is found that the endocannabinoid anandamide and the cannabinoid WIN 55,212-2 tin can alter the overall response to sympathetic nervus stimulation, which indicates that prejunctional CB1 receptors mediate the sympatho-inhibitory action. Thus cannabinoids tin inhibit both the noradrenergic and purinergic components of sympathetic neurotransmission.[23]
Central nervous system [edit]

Brain areas containing noradrenergic neurons.
The noradrenergic neurons in the encephalon grade a neurotransmitter system, that, when activated, exerts effects on large areas of the encephalon. The effects are manifested in alertness, arousal, and readiness for action.
Noradrenergic neurons (i.e., neurons whose principal neurotransmitter is norepinephrine) are comparatively few in number, and their jail cell bodies are confined to a few relatively small encephalon areas, but they send projections to many other encephalon areas and exert powerful effects on their targets. These noradrenergic jail cell groups were commencement mapped in 1964 by Annica Dahlström and Kjell Fuxe, who assigned them labels starting with the letter "A" (for "aminergic").[24] In their scheme, areas A1 through A7 contain the neurotransmitter norepinephrine (A8 through A14 contain dopamine). Noradrenergic cell group A1 is located in the caudal ventrolateral part of the medulla, and plays a role in the control of body fluid metabolism.[25] Noradrenergic cell grouping A2 is located in a brainstem area called the solitary nucleus; these cells take been implicated in a diverseness of responses, including control of food intake and responses to stress.[26] Cell groups A5 and A7 projection mainly to the spinal cord.[27]
The about important source of norepinephrine in the encephalon is the locus coeruleus, which contains noradrenergic cell group A6 and adjoins cell grouping A4. The locus coeruleus is quite small in absolute terms—in primates it is estimated to contain around fifteen,000 neurons, less than one-millionth of the neurons in the encephalon—but it sends projections to every major office of the brain and also to the spinal cord.[28]
The level of activity in the locus coeruleus correlates broadly with vigilance and speed of reaction. LC activity is low during sleep and drops to virtually nothing during the REM (dreaming) land.[29] Information technology runs at a baseline level during wakefulness, but increases temporarily when a person is presented with whatever sort of stimulus that draws attention. Unpleasant stimuli such every bit pain, difficulty breathing, bladder amplification, heat or common cold generate larger increases. Extremely unpleasant states such as intense fear or intense pain are associated with very high levels of LC activeness.[28]
Norepinephrine released by the locus coeruleus affects encephalon function in a number of ways. It enhances processing of sensory inputs, enhances attending, enhances formation and retrieval of both long term and working memory, and enhances the ability of the brain to respond to inputs past changing the action pattern in the prefrontal cortex and other areas.[thirty] The control of arousal level is stiff enough that drug-induced suppression of the LC has a powerful sedating effect.[29]
There is peachy similarity between situations that actuate the locus coeruleus in the brain and situations that activate the sympathetic nervous arrangement in the periphery: the LC essentially mobilizes the encephalon for activity while the sympathetic system mobilizes the torso. It has been argued that this similarity arises because both are to a large degree controlled by the same brain structures, particularly a part of the brainstem chosen the nucleus gigantocellularis.[28]
Skin [edit]
Norepinephrine is also produced by Merkel cells which are part of the somatosensory system. It activates the afferent sensory neuron.[31]
Pharmacology [edit]
A large number of important drugs exert their effects past interacting with norepinephrine systems in the encephalon or body. Their uses include handling of cardiovascular problems, shock, and a diverseness of psychiatric conditions. These drugs are divided into: sympathomimetic drugs which mimic or enhance at least some of the furnishings of norepinephrine released past the sympathetic nervous system; sympatholytic drugs, in contrast, block at least some of the furnishings.[32] Both of these are large groups with diverse uses, depending on exactly which effects are enhanced or blocked.[32]
Norepinephrine itself is classified as a sympathomimetic drug: its effects when given past intravenous injection of increasing heart charge per unit and force and constricting claret vessels make it very useful for treating medical emergencies that involve critically low claret pressure.[32] Surviving Sepsis Campaign recommended norepinephrine as showtime line agent in treating septic shock which is unresponsive to fluid resuscitation, supplemented by vasopressin and epinephrine. Dopamine usage is restricted only to highly selected patients.[33]
Beta blockers [edit]
These are sympatholytic drugs that block the effects of beta adrenergic receptors while having lilliputian or no effect on blastoff receptors. They are sometimes used to treat high blood pressure, atrial fibrillation and congestive heart failure, but contempo reviews accept ended that other types of drugs are usually superior for those purposes.[34] [35] Beta blockers may exist a viable pick for other cardiovascular atmospheric condition, though, including angina and Marfan syndrome.[36] They are also widely used to treat glaucoma, most unremarkably in the course of eyedrops.[37] Because of their furnishings in reducing anxiety symptoms and tremor, they accept sometimes been used by entertainers, public speakers and athletes to reduce performance anxiety, although they are not medically approved for that purpose and are banned by the International Olympic Committee.[38] [39]
Even so, the usefulness of beta blockers is express by a range of serious side furnishings, including slowing of heart rate, a drop in blood pressure level, asthma, and reactive hypoglycemia.[37] The negative effects can exist particularly severe in people who suffer from diabetes.[34]
Blastoff blockers [edit]
These are sympatholytic drugs that block the effects of adrenergic blastoff receptors while having niggling or no event on beta receptors.[40] Drugs belonging to this group can have very different effects, however, depending on whether they primarily block alpha-one receptors, alpha-2 receptors, or both. Alpha-two receptors, as described elsewhere in this article, are often located on norepinephrine-releasing neurons themselves and have inhibitory effects on them; consequently, blockage of blastoff-2 receptors commonly results in an increase in norepinephrine release.[40] Alpha-1 receptors are usually located on target cells and accept excitatory effects on them; consequently, blockage of alpha-one receptors usually results in blocking some of the effects of norepinephrine.[xl] Drugs such every bit phentolamine that human action on both types of receptors tin produce a complex combination of both furnishings. In nearly cases when the term "blastoff blocker" is used without qualification, information technology refers to a selective alpha-1 adversary.
Selective alpha-ane blockers have a variety of uses. Since ane of their furnishings is to inhibit the contraction of the smooth musculus in the prostate, they are often used to treat symptoms of benign prostatic hyperplasia.[41] Alpha-blockers also probable assist people pass their kidney stones.[42] Their effects on the central nervous system make them useful for treating generalized feet disorder, panic disorder, and posttraumatic stress disorder.[43] They may, nonetheless, have significant side-effects, including a drib in blood pressure.[40]
Some antidepressants role partly as selective alpha-ii blockers, but the best-known drug in that form is yohimbine, which is extracted from the bark of the African yohimbe tree.[44] Yohimbine acts as a male potency enhancer, but its usefulness for that purpose is limited by serious side-effects including anxiety and insomnia.[44] Overdoses can cause a unsafe increase in claret pressure.[44] Yohimbine is banned in many countries, just in the United States, considering it is extracted from a establish rather than chemically synthesized, it is sold over the counter equally a nutritional supplement.[45]
Alpha-two agonists [edit]
These are sympathomimetic drugs that activate alpha-ii receptors or enhance their effects.[46] Because alpha-2 receptors are inhibitory and many are located presynaptically on norepinephrine-releasing cells, the net effect of these drugs is usually to reduce the amount of norepinephrine released.[46] Drugs in this group that are capable of inbound the encephalon oftentimes have potent sedating furnishings, due to their inhibitory effects on the locus coeruleus.[46] Clonidine, for instance, is used for the treatment of anxiety disorders and insomnia, and likewise as a sedative premedication for patients nigh to undergo surgery.[47] Xylazine, another drug in this grouping, is also a powerful sedative and is often used in combination with ketamine as a full general anaesthetic for veterinary surgery—in the U.s.a. information technology has not been approved for employ in humans.[48]
Stimulants and antidepressants [edit]
These are drugs whose primary effects are idea to be mediated by different neurotransmitter systems (dopamine for stimulants, serotonin for antidepressants), but many also increase levels of norepinephrine in the brain.[49] Amphetamine, for example, is a stimulant that increases release of norepinephrine also as dopamine.[50] Monoamine oxidase inhibitors are antidepressants that inhibit the metabolic degradation of norepinephrine besides as serotonin and dopamine.[51] In some cases it is difficult to distinguish the norepinephrine-mediated effects from the effects related to other neurotransmitters.[ commendation needed ]
Diseases and disorders [edit]
A number of important medical problems involve dysfunction of the norepinephrine system in the brain or trunk.
Sympathetic hyperactivation [edit]
Hyperactivation of the sympathetic nervous system is not a recognized status in itself, only information technology is a component of a number of conditions, likewise every bit a possible consequence of taking sympathomimetic drugs. It causes a distinctive set of symptoms including aches and pains, rapid heartbeat, elevated claret force per unit area, sweating, palpitations, anxiety, headache, paleness, and a drib in blood glucose. If sympathetic activity is elevated for an extended fourth dimension, it tin cause weight loss and other stress-related body changes.
The list of conditions that tin cause sympathetic hyperactivation includes severe encephalon injury,[52] spinal cord impairment,[53] heart failure,[54] loftier blood pressure,[55] kidney affliction,[56] and various types of stress.
Pheochromocytoma [edit]
A pheochromocytoma is a rarely occurring tumor of the adrenal medulla, caused either by genetic factors or sure types of cancer. The consequence is a massive increase in the corporeality of norepinephrine and epinephrine released into the bloodstream. The well-nigh obvious symptoms are those of sympathetic hyperactivation, including specially a ascension in blood pressure that can reach fatal levels. The most effective treatment is surgical removal of the tumor.
Stress [edit]
Stress, to a physiologist, ways any state of affairs that threatens the continued stability of the body and its functions.[57] Stress affects a wide multifariousness of body systems: the two about consistently activated are the hypothalamic-pituitary-adrenal axis and the norepinephrine system, including both the sympathetic nervous arrangement and the locus coeruleus-centered organisation in the brain.[57] Stressors of many types evoke increases in noradrenergic activity, which mobilizes the brain and body to see the threat.[57] Chronic stress, if continued for a long fourth dimension, can damage many parts of the body. A meaning role of the damage is due to the effects of sustained norepinephrine release, because of norepinephrine'due south full general function of directing resources abroad from maintenance, regeneration, and reproduction, and toward systems that are required for active movement. The consequences can include slowing of growth (in children), sleeplessness, loss of libido, gastrointestinal bug, impaired disease resistance, slower rates of injury healing, low, and increased vulnerability to addiction.[57]
ADHD [edit]
Attention deficit hyperactivity disorder is a psychiatric condition involving problems with attending, hyperactivity, and impulsiveness.[58] It is most commonly treated using stimulant drugs such as methylphenidate (Ritalin), whose primary effect is to increase dopamine levels in the brain, but drugs in this group besides more often than not increment encephalon levels of norepinephrine, and it has been difficult to make up one's mind whether these actions are involved in their clinical value. There is as well substantial evidence that many people with ADHD show biomarkers involving contradistinct norepinephrine processing.[59] Several drugs whose primary effects are on norepinephrine, including guanfacine, clonidine, and atomoxetine, accept been tried as treatments for ADHD, and found to have furnishings comparable to those of stimulants.[60] [61]
Autonomic failure [edit]
Several weather, including Parkinson's affliction, diabetes and and then-called pure autonomic failure, tin can cause a loss of norepinephrine-secreting neurons in the sympathetic nervous system. The symptoms are widespread, the nearly serious being a reduction in heart rate and an extreme drop in resting blood pressure, making it impossible for severely affected people to stand up for more than than a few seconds without fainting. Handling can involve dietary changes or drugs.[62]
Comparative biology and evolution [edit]
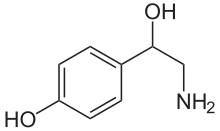
Chemical structure of octopamine, which serves as the homologue of norepinephrine in many invertebrate species
Norepinephrine has been reported to exist in a broad diverseness of animal species, including protozoa,[63] placozoa and cnidaria (jellyfish and related species),[64] but not in ctenophores (comb jellies), whose nervous systems differ greatly from those of other animals.[65] It is generally present in deuterostomes (vertebrates, etc.), but in protostomes (arthropods, molluscs, flatworms, nematodes, annelids, etc.) it is replaced by octopamine, a closely related chemical with a closely related synthesis pathway.[63] In insects, octopamine has alerting and activating functions that correspond (at least roughly) with the functions of norepinephrine in vertebrates.[66] It has been argued that octopamine evolved to replace norepinephrine rather than vice versa; however, the nervous arrangement of amphioxus (a primitive chordate) has been reported to contain octopamine but non norepinephrine, which presents difficulties for that hypothesis.[63]
History [edit]
Early in the twentieth century Walter Cannon, who had popularized the idea of a sympathoadrenal system preparing the body for fight and flight, and his colleague Arturo Rosenblueth developed a theory of two sympathins, sympathin E (excitatory) and sympathin I (inhibitory), responsible for these deportment.[67] The Belgian pharmacologist Zénon Bacq as well equally Canadian and US-American pharmacologists between 1934 and 1938 suggested that noradrenaline might be a sympathetic transmitter.[67] In 1939, Hermann Blaschko and Peter Holtz independently identified the biosynthetic mechanism for norepinephrine in the vertebrate body.[68] [69] In 1945 Ulf von Euler published the first of a series of papers that established the role of norepinephrine as a neurotransmitter.[seventy] He demonstrated the presence of norepinephrine in sympathetically innervated tissues and brain, and adduced testify that it is the sympathin of Cannon and Rosenblueth. Stanley Peart was the first to demonstrate the release of noradrenaline later the stimulation of sympathetic fretfulness.
References [edit]
- ^ Andersen, A. One thousand. (1975). "Structural Studies of Metabolic Products of Dopamine. Iv. Crystal and Molecular Structure of (−)-Noradrenaline". Acta Chem. Scand. 29b (8): 871–876. doi:10.3891/acta.chem.scand.29b-0871. PMID 1202890.
- ^ Aronson JK (February 2000). "'Where name and paradigm meet'—the argument for 'adrenaline'". British Medical Periodical. 320 (7233): 506–9. doi:x.1136/bmj.320.7233.506. PMC1127537. PMID 10678871.
- ^ "(-)-noradrenaline". IUPHAR database. International Marriage of Bones and Clinical Pharmacology. Retrieved 2 Jan 2016.
- ^ a b "Norepinephrine". PubChem . Retrieved six November 2015.
- ^ Gaddum JH (June 1956). "The Prefix 'Nor' in Chemical Nomenclature". Nature. 177 (1046): 1046. Bibcode:1956Natur.177.1046G. doi:x.1038/1771046b0. S2CID 4284979.
- ^ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (three): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ Lindemann 50, Hoener MC (May 2005). "A renaissance in trace amines inspired past a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Periodical of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ^ a b c d due east f g Musacchio JM (2013). "Chapter i: Enzymes involved in the biosynthesis and degradation of catecholamines". In Iverson L (ed.). Biochemistry of Biogenic Amines. Springer. pp. 1–35. ISBN978-i-4684-3171-1.
- ^ Griffith RK (2013). "Chapter 10: Adrenergic Receptors and Drugs Affecting Adrenergic Neurotransmission". In Lemke TL, Williams DA, Zito SW, Roche VF (eds.). Foye's Principles of Medicinal Chemistry (7th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 343. ISBN978-1-60913-345-0.
- ^ a b c d due east f yard h i Rang HP, Ritter JM, Blossom R, Henderson One thousand (2014). "Chapter 14: Noradrenergic transmission". Rang & Dale's Pharmacology. Elsevier Health Sciences. pp. 177–196. ISBN978-0-7020-5497-6.
- ^ a b Eiden LE, Schäfer MK, Weihe East, Schütz B (2004). "The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine". Pflügers Arch. 447 (5): 636–40. doi:10.1007/s00424-003-1100-five. PMID 12827358. S2CID 20764857.
- ^ Torres GE, Gainetdinov RR, Caron MG (2003). "Plasma membrane monoamine transporters: construction, regulation and office". Nature Reviews Neuroscience. 4 (1): thirteen–25. doi:10.1038/nrn1008. PMID 12511858. S2CID 21545649.
- ^ a b c Hamill RW, Shapiro RE, Vizzard MA (2012). "Peripheral Autonomic Nervous System". In Robertson D, Biaggioni I, et al. (eds.). Primer on the Autonomic Nervous System. Academic Press. pp. 17–xx. ISBN978-0-12-386525-0.
- ^ a b Schacter D, Gilbert D, Wegner D, Hood B (2011). Psychology: European Edition. Palgrave Macmillan. p. 93. ISBN978-0-230-34367-two.
- ^ Dartt DA (May 2009). "Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases". Progress in Retinal and Centre Research. 28 (3): 155–77. doi:10.1016/j.preteyeres.2009.04.003. PMC3652637. PMID 19376264.
- ^ Tank AW, Lee Wong D (Jan 2015). "Peripheral and central furnishings of circulating catecholamines". Comprehensive Physiology. Vol. 5. pp. 1–15. doi:10.1002/cphy.c140007. ISBN9780470650714. PMID 25589262.
- ^ Bahler 50, Molenaars RJ, Verberne HJ, Holleman F (September 2015). "Role of the autonomic nervous system in activation of human brown adipose tissue: A review of the literature". Diabetes & Metabolism. 41 (6): 437–445. doi:x.1016/j.diabet.2015.08.005. PMID 26404650.
- ^ Kenney MJ, Ganta CK (July 2014). "Autonomic nervous system and immune organization interactions". Comprehensive Physiology. Vol. 4. pp. 1177–200. doi:10.1002/cphy.c130051. ISBN9780470650714. PMC4374437. PMID 24944034.
- ^ Chistiakov DA, Ashwell KW, Orekhov AN, Bobryshev YV (2015). "Innervation of the arterial wall and its modification in atherosclerosis". Auton Neurosci. 193: 7–eleven. doi:10.1016/j.autneu.2015.06.005. PMID 26164815. S2CID 8150131.
- ^ a b c d e Thorp AA, Schlaich MP (2015). "Relevance of Sympathetic Nervous Arrangement Activation in Obesity and Metabolic Syndrome". J Diabetes Res. 2015: 1–11. doi:ten.1155/2015/341583. PMC4430650. PMID 26064978.
- ^ Konturek SJ, Konturek JW, Pawlik T, Brzozowski T (2004). "Brain-gut axis and its role in the control of nutrient intake" (PDF). J. Physiol. Pharmacol. 55 (one Pt 2): 137–54. PMID 15082874.
- ^ Pakdeechote P, Dunn WR, Ralevic V (November 2007). "Cannabinoids inhibit noradrenergic and purinergic sympathetic cotransmission in the rat isolated mesenteric arterial bed". British Journal of Pharmacology. 152 (v): 725–33. doi:10.1038/sj.bjp.0707397. PMC2190027. PMID 17641668.
- ^ Dahlstroem A, Fuxe G (1964). "Evidence for the beingness of monoamine-containing neurons in the primal nervous organisation. I. Demonstration of monoamines in the cell bodies of brain stem neurons". Acta Physiologica Scandinavica. Supplementum. 232 (Supplement 232): 1–55. PMID 14229500.
- ^ Antunes-Rodrigues J, de Castro M, Elias LL, Valença MM, McCann SM (Jan 2004). "Neuroendocrine command of trunk fluid metabolism" (PDF). Physiological Reviews. 84 (1): 169–208. doi:10.1152/physrev.00017.2003. PMID 14715914. S2CID 14046. Archived from the original (PDF) on 6 March 2019.
- ^ Rinaman L (February 2011). "Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cerebral, and behavioral functions". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 300 (ii): R222–35. doi:x.1152/ajpregu.00556.2010. PMC3043801. PMID 20962208.
- ^ Bruinstroop Due east, Cano Thou, Vanderhorst VG, Cavalcante JC, Wirth J, Sena-Esteves Thou, Saper CB (June 2012). "Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic jail cell groups in rats". The Journal of Comparative Neurology. 520 (ix): 1985–2001. doi:10.1002/cne.23024. PMC3508755. PMID 22173709.
- ^ a b c Sara SJ, Bouret Due south (2012). "Orienting and reorienting: the locus coeruleus mediates cognition through arousal". Neuron. 76 (1): 130–41. doi:10.1016/j.neuron.2012.09.011. PMID 23040811.
- ^ a b Berridge CW, Schmeichel BE, España RA (2012). "Noradrenergic modulation of wakefulness/arousal". Sleep Med Rev. 16 (ii): 187–97. doi:10.1016/j.smrv.2011.12.003. PMC3278579. PMID 22296742.
- ^ Sara SJ (2015). "Locus Coeruleus in time with the making of memories". Curr. Opin. Neurobiol. 35: 87–94. doi:10.1016/j.conb.2015.07.004. PMID 26241632. S2CID 206952441.
- ^ Feng J, Hu H (December 2019). "A novel role player in the field: Merkel disc in affect, crawling and pain". Experimental Dermatology. 28 (12): 1412–1415. doi:x.1111/exd.13945. PMC6800577. PMID 31001848.
- ^ a b c Gardenhire DS (2013). Rau's Respiratory Care Pharmacology. Elsevier Health Sciences. p. 88. ISBN978-0-323-27714-3.
- ^ Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli Chiliad, Ferrer R, et al. (March 2017). "Surviving Sepsis Entrada: International Guidelines for Management of Sepsis and Septic Shock: 2016" (PDF). Critical Care Medicine. 45 (3): 486–552. doi:10.1097/CCM.0000000000002255. PMID 28098591. S2CID 52827184.
Nosotros recommend norepinephrine as the commencement-choice vasopressor (strong recommendation, moderate quality of evidence).
[ permanent dead link ] - ^ a b Deedwania PC (2015). "Management of Patients With Stable Angina and Blazon ii Diabetes". Rev Cardiovasc Med. 16 (two): 105–13. PMID 26198557.
- ^ Mareev Y, Cleland JG (2015). "Should β-Blockers Exist Used in Patients With Middle Failure and Atrial Fibrillation?". Clin Ther. 37 (10): 2215–24. doi:10.1016/j.clinthera.2015.08.017. PMID 26391145.
- ^ Kumar A, Agarwal S (2014). "Marfan syndrome: An eyesight of syndrome". Meta Gene. two: 96–105. doi:10.1016/j.mgene.2013.10.008. PMC4287801. PMID 25606393.
- ^ a b Inoue K (2014). "Managing adverse effects of glaucoma medications". Clin Ophthalmol. eight: 903–thirteen. doi:10.2147/OPTH.S44708. PMC4025938. PMID 24872675.
- ^ Brugués AO (2011). "Music performance feet-part 2. a review of treatment options". Med Probl Perform Art. 26 (3): 164–71. doi:10.21091/mppa.2011.3026. PMID 21987072.
- ^ Fitch Thou (2012). "Proscribed drugs at the Olympic Games: permitted employ and misuse (doping) by athletes". Clin Med. 12 (3): 257–sixty. doi:10.7861/clinmedicine.12-three-257. PMC4953490. PMID 22783779.
- ^ a b c d Lilley LL, Collins SR, Snyder JS (2014). Pharmacology and the Nursing Process (7th ed.). Elsevier Wellness Sciences. pp. 313–316. ISBN978-0-323-29361-7.
- ^ Hollingsworth JM, Wilt TJ (Baronial 2014). "Lower urinary tract symptoms in men". BMJ. 349: g4474. doi:10.1136/bmj.g4474. PMC4688452. PMID 25125424.
- ^ Campschroer T, Zhu 10, Vernooij RW, Lock MT (Apr 2018). "Alpha-blockers as medical expulsive therapy for ureteral stones". The Cochrane Database of Systematic Reviews. 2018 (4): CD008509. doi:10.1002/14651858.CD008509.pub3. PMC6494465. PMID 29620795.
- ^ Dark-green B (July 2014). "Prazosin in the treatment of PTSD". Journal of Psychiatric Practice. twenty (iv): 253–nine. doi:ten.1097/01.pra.0000452561.98286.1e. PMID 25036580. S2CID 40069887.
- ^ a b c Corazza O, Martinotti K, Santacroce R, Chillemi E, Di Giannantonio M, Schifano F, Cellek S (2014). "Sexual enhancement products for sale online: raising awareness of the psychoactive effects of yohimbine, maca, horny goat weed, and Ginkgo biloba". Biomed Res Int. 2014: ane–13. doi:x.1155/2014/841798. PMC4082836. PMID 25025070.
- ^ EFSA Panel on Food Additives and Nutrient Sources Added to Food (2013). "Scientific Opinion on the evaluation of the safety in utilize of Yohimbe". EFSA Journal. 11 (seven): 3302. doi:ten.2903/j.efsa.2013.3302.
- ^ a b c Lemke KA (2004). "Perioperative use of selective blastoff-2 agonists and antagonists in small animals". Tin. Vet. J. 45 (six): 475–eighty. PMC548630. PMID 15283516.
- ^ Belkin MR, Schwartz TL (2015). "Blastoff-2 receptor agonists for the handling of posttraumatic stress disorder". Drugs in Context. 4: 1–v. doi:10.7573/dic.212286. PMC4544272. PMID 26322115.
- ^ Greene SA, Thurmon JC (1988). "Xylazine—a review of its pharmacology and utilize in veterinarian medicine". J. Vet. Pharmacol. Ther. 11 (4): 295–313. doi:10.1111/j.1365-2885.1988.tb00189.x. PMID 3062194.
- ^ Sofuoglu M, Sewell RA (Apr 2009). "Norepinephrine and stimulant addiction". Addiction Biology. 14 (2): 119–29. doi:10.1111/j.1369-1600.2008.00138.x. PMC2657197. PMID 18811678.
- ^ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present--a pharmacological and clinical perspective". Periodical of Psychopharmacology. 27 (vi): 479–96. doi:ten.1177/0269881113482532. PMC3666194. PMID 23539642.
- ^ Finberg JP, Rabey JM (2016). "Inhibitors of MAO-A and MAO-B in Psychiatry and Neurology". Frontiers in Pharmacology. 7: 340. doi:10.3389/fphar.2016.00340. PMC5067815. PMID 27803666.
Selective inhibition of MAO-A leads to increased levels of neurotransmitter inside noradrenergic (NA-ergic) and 5-HT-ergic neurons of the CNS, and clinical antidepressant action, while inhibition of MAO-B leads to increased levels of DA in the Parkinsonian brain...
- ^ Lump D, Moyer G (2014). "Paroxysmal sympathetic hyperactivity after severe brain injury". Curr Neurol Neurosci Rep. 14 (11): 494. doi:10.1007/s11910-014-0494-0. PMID 25220846. S2CID 10849388.
- ^ Amzallag Yard (1993). "Autonomic hyperreflexia". Int Anesthesiol Clin. 31 (1): 87–102. doi:10.1097/00004311-199331010-00009. PMID 8440534. S2CID 32173637.
- ^ McCrink KA, Brill A, Lymperopoulos A (2015). "Adrenal G protein-coupled receptor kinase-2 in regulation of sympathetic nervous organization activeness in heart failure". World J Cardiol. 7 (9): 539–43. doi:ten.4330/wjc.v7.i9.539. PMC4577680. PMID 26413230.
- ^ Malpas SC (2010). "Sympathetic nervous system overactivity and its part in the development of cardiovascular disease". Physiol. Rev. 90 (2): 513–57. doi:10.1152/physrev.00007.2009. PMID 20393193.
- ^ Ksiazek A, Załuska Due west (2008). "Sympathetic overactivity in uremia". J Ren Nutr. 18 (1): 118–21. doi:10.1053/j.jrn.2007.x.024. PMID 18089457.
- ^ a b c d Chrousos GP (2009). "Stress and disorders of the stress system". Nat Rev Endocrinol. 5 (seven): 374–81. doi:10.1038/nrendo.2009.106. PMID 19488073. S2CID 2259451.
- ^ Kooij SJ, Bejerot South, et al. (2010). "European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD". BMC Psychiatry. 10: 67. doi:10.1186/1471-244X-10-67. PMC2942810. PMID 20815868.
- ^ Faraone SV, Bonvicini C, Scassellati C (2014). "Biomarkers in the diagnosis of ADHD--promising directions". Curr Psychiatry Rep. 16 (11): 497. doi:10.1007/s11920-014-0497-one. PMID 25298126. S2CID 36702503.
- ^ Bello NT (2015). "Clinical utility of guanfacine extended release in the handling of ADHD in children and adolescents". Patient Prefer Adherence. 9: 877–85. doi:x.2147/PPA.S73167. PMC4494608. PMID 26170637.
- ^ Clemow DB, Bushe CJ (2015). "Atomoxetine in patients with ADHD: A clinical and pharmacological review of the onset, trajectory, duration of response and implications for patients". J. Psychopharmacol. (Oxford). 29 (12): 1221–xxx. doi:ten.1177/0269881115602489. PMID 26349559. S2CID 22649093.
- ^ Shibao C, Okamoto Fifty, Biaggioni I (2012). "Pharmacotherapy of autonomic failure". Pharmacol. Ther. 134 (3): 279–86. doi:10.1016/j.pharmthera.2011.05.009. PMC3358114. PMID 21664375.
- ^ a b c Pflüger HJ, Stevensonb PA (2005). "Evolutionary aspects of octopaminergic systems with emphasis on arthropods". Arthropod Structure & Development. 34 (3): 379–396. doi:10.1016/j.asd.2005.04.004.
- ^ Kass-Simon One thousand, Pierobon P (2007). "Cnidarian chemical neurotransmission, an updated overview". Comp. Biochem. Physiol. A. 146 (1): 9–25. doi:10.1016/j.cbpa.2006.09.008. PMID 17101286.
- ^ Moroz LL (2015). "Convergent evolution of neural systems in ctenophores". J. Exp. Biol. 218 (Pt 4): 598–611. doi:x.1242/jeb.110692. PMC4334147. PMID 25696823.
- ^ Verlinden H, Vleugels R, Marchal Due east, Badisco 50, Pflüger HJ, Blenau W, Broeck JV (2010). "The role of octopamine in locusts and other arthropods". J. Insect Physiol. 56 (8): 854–67. doi:x.1016/j.jinsphys.2010.05.018. PMID 20621695.
- ^ a b Bacq ZM (1983). "Chemical manual of nervus impulses". In Parnham MJ, Bruinvels J (eds.). Discoveries in Pharmacology, Book 1. Amsterdam: Elsevier. pp. 49–103. ISBN978-0-444-80493-viii.
- ^ Herman Blaschko (1987). "A half-century of research on catecholamine biosynthesis". Journal of Applied Cardiology: 171–183.
- ^ P. Holtz (1939). "Dopadecarboxylase". Dice Naturwissenschaften (in German). 27 (43): 724–725. Bibcode:1939NW.....27..724H. doi:10.1007/bf01494245.
- ^ von Euler Usa (1945). "A sympathomimetic pressor substance in animal organ extracts". Nature. 156 (3949): 18–19. Bibcode:1945Natur.156...18V. doi:10.1038/156018b0. S2CID 4100718.
caldwellambee1962.blogspot.com
Source: https://en.wikipedia.org/wiki/Norepinephrine
0 Response to "Clip Art Vesicles Clip Art of Cell Wall in Plant Cells"
Post a Comment